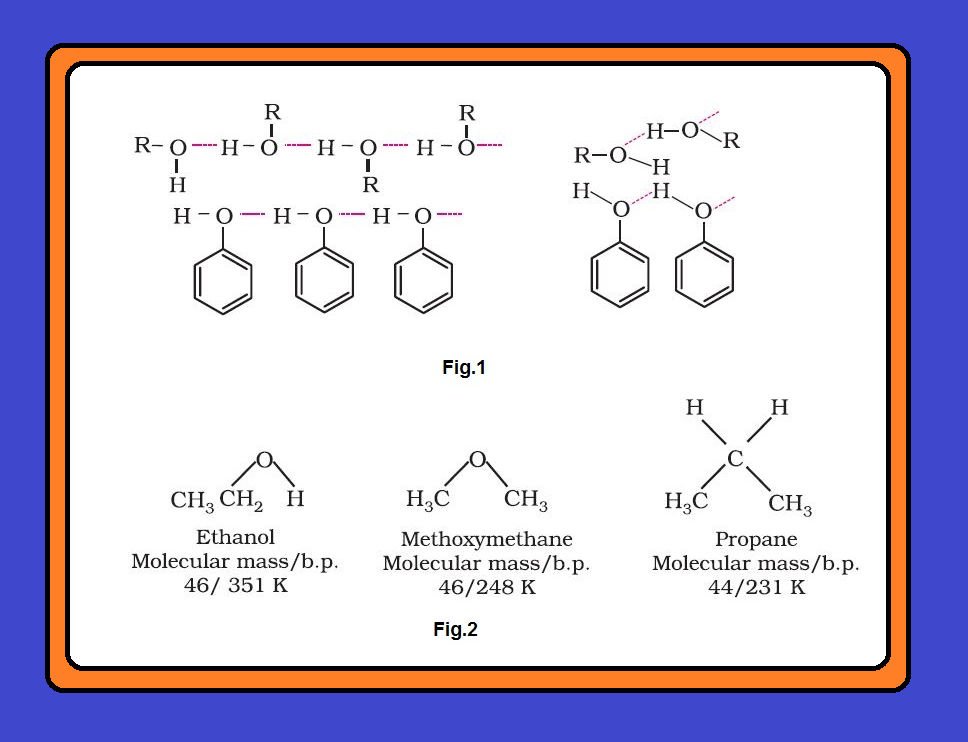
(i) `color{green}("Reaction with Metals ")` : Alcohols and phenols react with active metals such as sodium, potassium and aluminium to yield corresponding alkoxides/phenoxides and hydrogen. See fig.1.
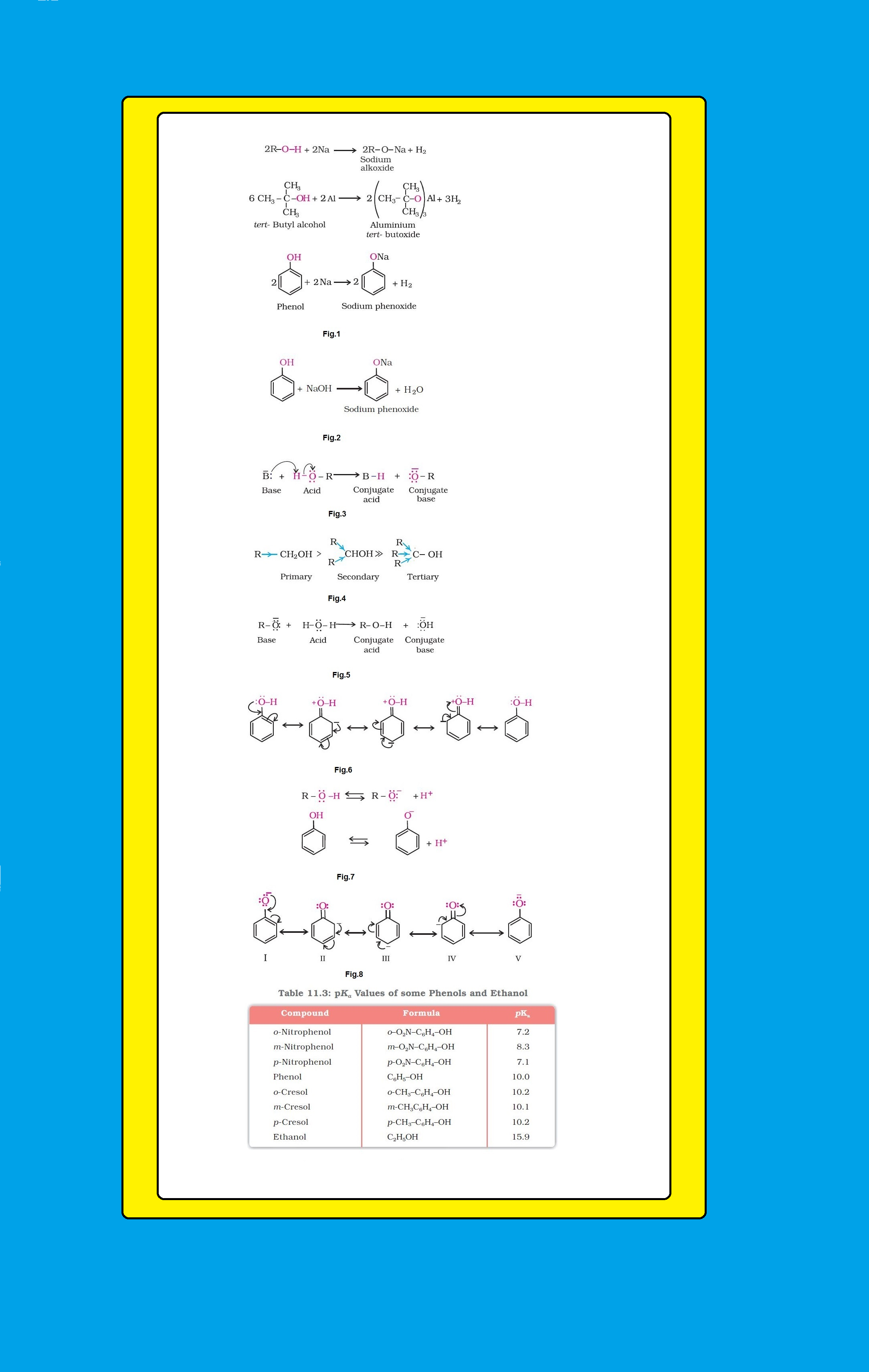
`color{red}(2R - O - H +2Na → undersettext(Sodium alkoxide)(2R - O - Na+H_2))`
● In addition to this, phenols react with aqueous sodium hydroxide to form sodium phenoxides. See fig.2.
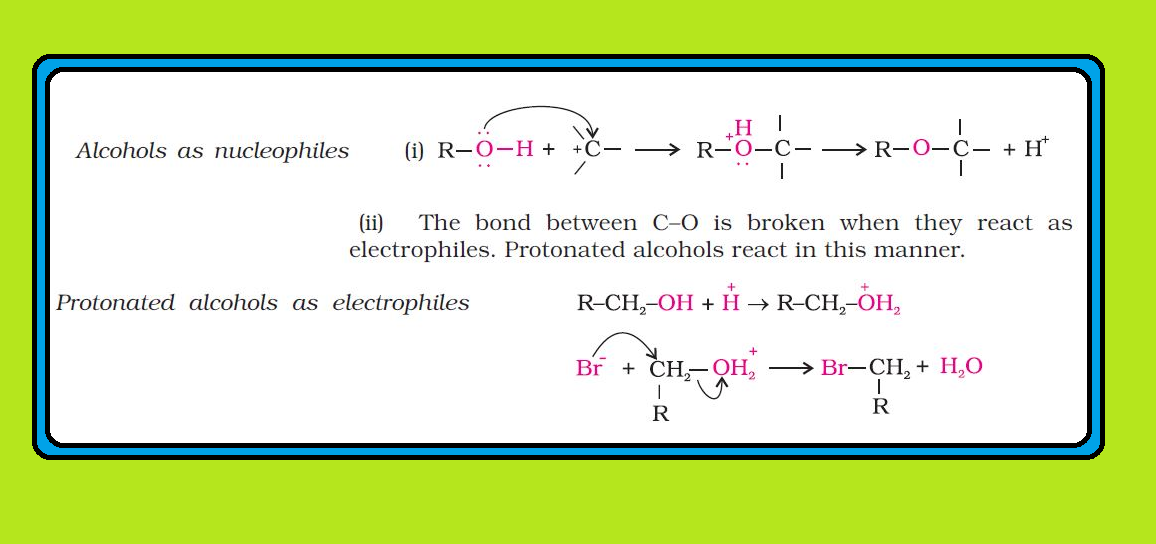
● The above reactions show that alcohols and phenols are acidic in nature. In fact, alcohols and phenols are Brönsted acids i.e., they can donate a proton to a stronger base (`B:`). See fig.3.
(ii) `color{green}("Acidity of Alcohols ")` : The acidic character of alcohols is due to the polar nature of `color{red}(O–H)` bond.
● An electron-releasing group `color{red}(–CH_3, –C_2H_5)` increases electron density on oxygen tending to decrease the polarity of `color{red}(O-H)` bond. This decreases the acid strength.
● For this reason, the acid strength of alcohols decreases in the following order as shown in fig.4.
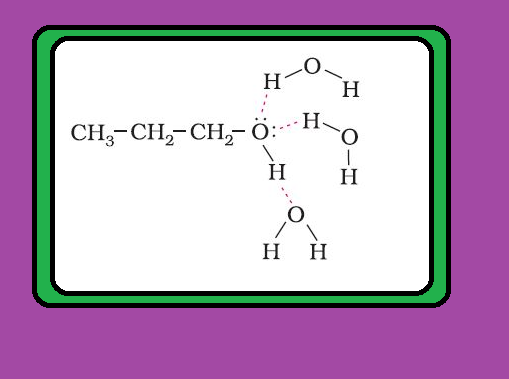
● Alcohols are, however, weaker acids than water. This can be illustrated by the reaction of water with an alkoxide. See fig.5.
● This reaction shows that water is a better proton donor (i.e., stronger acid) than alcohol.
● Also, in the above reaction, we note that an alkoxide ion is a better proton acceptor than hydroxide ion, which suggests that alkoxides are stronger bases (sodium ethoxide is a stronger base than sodium hydroxide).
● Alcohols act as Bronsted bases as well. It is due to the presence of unshared electron pairs on oxygen, which makes them proton acceptors.
(iii) `color{green}("Acidity of Phenols ")` : The reactions of phenol with metals (e.g., sodium, aluminium) and sodium hydroxide indicate its acidic nature.
● The hydroxyl group, in phenol is directly attached to the `color{red}(sp^2)` hybridised carbon of benzene ring which acts as an electron withdrawing group.
● Due to this, the charge distribution in phenol molecule, as depicted in its resonance structures, causes the oxygen of `color{red}(–OH)` group to be positive. See fig.6.
● The reaction of phenol with aqueous sodium hydroxide indicates that phenols are stronger acids than alcohols and water.
`=>` Let us examine how a compound in which hydroxyl group attached to an aromatic ring is more acidic than the one in which hydroxyl group is attached to an alkyl group. The ionisation of an alcohol and a phenol takes place as shown in fig.7.
● Due to the higher electronegativity of `color{red}(sp^2)` hybridised carbon of phenol to which `color{red}(–OH)` is attached, electron density decreases on oxygen. This increases the polarity of `color{red}(O–H)` bond and results in an increase in ionisation of phenols than that of alcohols.
`=>` Now let us examine the stabilities of alkoxide and phenoxide ions. In alkoxide ion, the negative charge is localised on oxygen while in phenoxide ion, the charge is delocalised.
● The delocalisation of negative charge (structures I-V) makes phenoxide ion more stable and favours the ionisation of phenol.
● Although there is also charge delocalisation in phenol, its resonance structures have charge separation due to which the phenol molecule is less stable than phenoxide ion. See fig.8.
`=>` In substituted phenols, the presence of electron withdrawing groups such as nitro group, enhances the acidic strength of phenol. This effect is more pronounced when such a group is present at ortho and para positions. It is due to the effective delocalisation of negative charge in phenoxide ion.
● On the other hand, electron releasing groups, such as alkyl groups, in general, do not favour the formation of phenoxide ion resulting in decrease in acid strength. Cresols, for example, are less acidic than phenol.
(i) `color{green}("Reaction with Metals ")` : Alcohols and phenols react with active metals such as sodium, potassium and aluminium to yield corresponding alkoxides/phenoxides and hydrogen. See fig.1.
`color{red}(2R - O - H +2Na → undersettext(Sodium alkoxide)(2R - O - Na+H_2))`
● In addition to this, phenols react with aqueous sodium hydroxide to form sodium phenoxides. See fig.2.
● The above reactions show that alcohols and phenols are acidic in nature. In fact, alcohols and phenols are Brönsted acids i.e., they can donate a proton to a stronger base (`B:`). See fig.3.
(ii) `color{green}("Acidity of Alcohols ")` : The acidic character of alcohols is due to the polar nature of `color{red}(O–H)` bond.
● An electron-releasing group `color{red}(–CH_3, –C_2H_5)` increases electron density on oxygen tending to decrease the polarity of `color{red}(O-H)` bond. This decreases the acid strength.
● For this reason, the acid strength of alcohols decreases in the following order as shown in fig.4.
● Alcohols are, however, weaker acids than water. This can be illustrated by the reaction of water with an alkoxide. See fig.5.
● This reaction shows that water is a better proton donor (i.e., stronger acid) than alcohol.
● Also, in the above reaction, we note that an alkoxide ion is a better proton acceptor than hydroxide ion, which suggests that alkoxides are stronger bases (sodium ethoxide is a stronger base than sodium hydroxide).
● Alcohols act as Bronsted bases as well. It is due to the presence of unshared electron pairs on oxygen, which makes them proton acceptors.
(iii) `color{green}("Acidity of Phenols ")` : The reactions of phenol with metals (e.g., sodium, aluminium) and sodium hydroxide indicate its acidic nature.
● The hydroxyl group, in phenol is directly attached to the `color{red}(sp^2)` hybridised carbon of benzene ring which acts as an electron withdrawing group.
● Due to this, the charge distribution in phenol molecule, as depicted in its resonance structures, causes the oxygen of `color{red}(–OH)` group to be positive. See fig.6.
● The reaction of phenol with aqueous sodium hydroxide indicates that phenols are stronger acids than alcohols and water.
`=>` Let us examine how a compound in which hydroxyl group attached to an aromatic ring is more acidic than the one in which hydroxyl group is attached to an alkyl group. The ionisation of an alcohol and a phenol takes place as shown in fig.7.
● Due to the higher electronegativity of `color{red}(sp^2)` hybridised carbon of phenol to which `color{red}(–OH)` is attached, electron density decreases on oxygen. This increases the polarity of `color{red}(O–H)` bond and results in an increase in ionisation of phenols than that of alcohols.
`=>` Now let us examine the stabilities of alkoxide and phenoxide ions. In alkoxide ion, the negative charge is localised on oxygen while in phenoxide ion, the charge is delocalised.
● The delocalisation of negative charge (structures I-V) makes phenoxide ion more stable and favours the ionisation of phenol.
● Although there is also charge delocalisation in phenol, its resonance structures have charge separation due to which the phenol molecule is less stable than phenoxide ion. See fig.8.
`=>` In substituted phenols, the presence of electron withdrawing groups such as nitro group, enhances the acidic strength of phenol. This effect is more pronounced when such a group is present at ortho and para positions. It is due to the effective delocalisation of negative charge in phenoxide ion.
● On the other hand, electron releasing groups, such as alkyl groups, in general, do not favour the formation of phenoxide ion resulting in decrease in acid strength. Cresols, for example, are less acidic than phenol.